R&D Trends in MoCoD-A: What Biopharma Needs to Know
Share this Post to earn Money ( Upto ₹100 per 1000 Views )
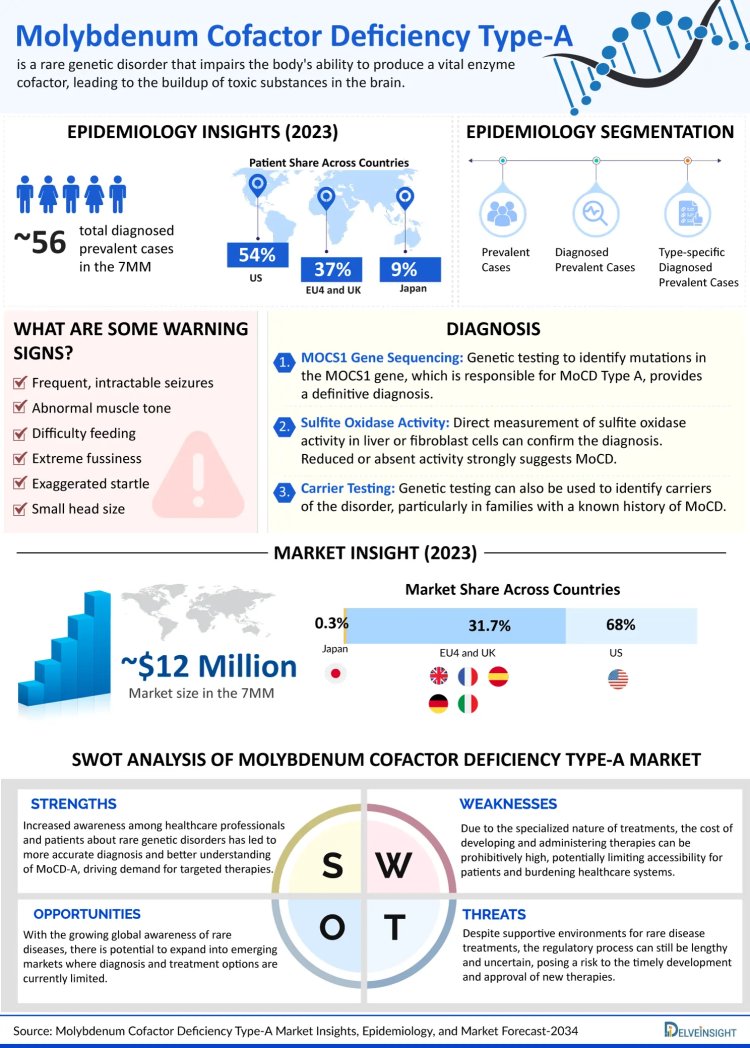
Molybdenum Cofactor Deficiency Type-A (MoCoD-A) is an ultra-rare, inherited metabolic disorder that manifests early in life with severe neurological symptoms. Caused by a deficiency in sulfite oxidase, a crucial molybdenum-dependent enzyme, this condition leads to toxic sulfite accumulation. Common features include intractable seizures, microcephaly, exaggerated startle reflexes, and profound developmental delays, often culminating in death during early childhood. The debilitating nature of this condition, coupled with its rarity, creates significant barriers to diagnosis, treatment, and research. For a visual summary of the MoCoD-A landscape, you can view the detailed infographic here: Explore MoCoD-A Visual Insights.
MoCoD-A Epidemiology Across Key Markets
Across the 7MM (United States, EU4, UK, and Japan), the epidemiological segmentation of MoCoD-A includes total prevalent cases, diagnosed prevalent cases, and type-specific data. In 2023, an estimated 270 diagnosed prevalent cases were reported, with the US accounting for approximately 30—a figure projected to increase with rising awareness and improved diagnostic capabilities. The EU4 and UK collectively accounted for about 20 cases, while Japan represented around 9% of the total diagnosed population. Interestingly, Spain reported the fewest cases among the EU4 countries. For a country-wise breakdown and forecasts, check out this comprehensive research coverage: Access Full MoCoD-A Market Report.
Market Size and Current Barriers
The MoCoD-A market across these seven major markets reached an estimated USD 12.20 million in 2023. Despite its modest size, this market reflects increasing efforts to improve clinical management and therapeutic access. However, several obstacles remain—including limited awareness, the high cost of genetic testing, and the absence of multiple effective treatments. These challenges delay diagnosis and complicate care, especially in low-resource regions. To dive deeper into the market trends and limitations shaping the future of MoCoD-A treatment, click here to review the latest market insights: Delve into MoCoD-A Market Dynamics.
Current Therapeutic Landscape and Future Outlook
At present, Nulibry (fosdenopterin) developed by BridgeBio Pharma remains the only FDA-approved treatment for MoCoD-A, offering a critical but limited option for patients. The rarity of the condition has deterred broader pharmaceutical investment, and the path to early diagnosis remains constrained by complex and expensive testing procedures. Still, with growing clinical interest and emerging research, the outlook is cautiously optimistic. Key players such as BridgeBio Pharma are expected to drive future developments in this underserved area. For an in-depth overview of market drivers, key companies, and upcoming advancements, visit this detailed report: Get the Full MoCoD-A Market Analysis.















