In-depth Report on Acetazolamide (Diamox) Production Process with Cost Analysis
The Acetazolamide (Diamox) Production Process with Cost Analysis report offers a comprehensive exploration of the methods and economic factors involved in the manufacturing of Acetazolamide, known commercially as Diamox.
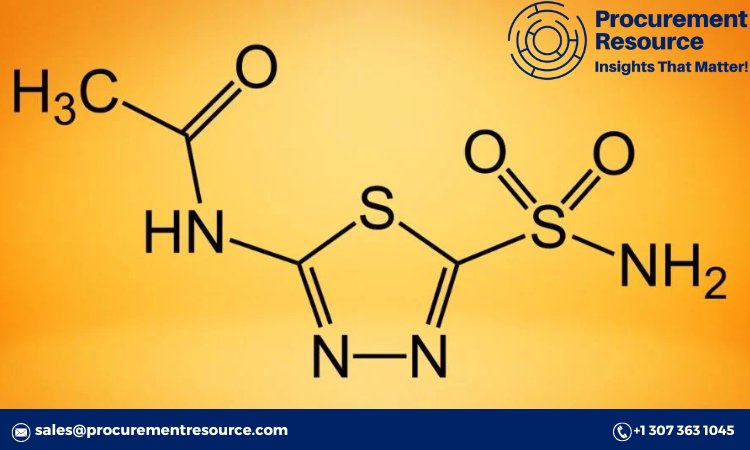
Introduction
The Acetazolamide (Diamox) Production Process with Cost Analysis report offers a comprehensive exploration of the methods and economic factors involved in the manufacturing of Acetazolamide, known commercially as Diamox. This medication is crucial in treating a variety of conditions, including glaucoma, epilepsy, altitude sickness, and certain types of edema. Our detailed report examines the production processes, assesses market dynamics, evaluates cost structures, and explores raw material requirements, providing essential insights for stakeholders in the pharmaceutical industry.
Request Free Sample - https://www.procurementresource.com/production-cost-report-store/acetazolamide/request-sample
Procurement Resource Assessment of the Acetazolamide (Diamox) Production Process
Conducting a thorough procurement resource assessment is fundamental for any entity involved in the production of Acetazolamide. This evaluation covers multiple key aspects:
-
Raw Material Sourcing: The primary raw materials for Acetazolamide production include acetic anhydride, ethyl cyanoacetate, and aromatic amines. Ensuring a reliable and cost-effective supply chain for these materials is critical to maintain uninterrupted production and manage costs effectively.
-
Production Technologies: The synthesis of Acetazolamide involves specific chemical reactions, including condensation and cyclization processes that require precision and control. The choice of technology significantly impacts production efficiency, product quality, and environmental sustainability.
-
Supply Chain Management: Effective management of the supply chain, including the procurement, storage, and transportation of raw materials and finished products, is vital for minimizing operational disruptions and ensuring timely delivery.
-
Regulatory Compliance: Adhering to pharmaceutical manufacturing regulations is essential. Compliance with Good Manufacturing Practices (GMP) and other industry standards guarantees the production of high-quality, safe, and effective Acetazolamide.
Understanding Acetazolamide (Diamox)
Acetazolamide, marketed as Diamox, is a versatile medication with several important medical applications. Here's a closer look at its properties and uses:
-
Chemical Properties: Acetazolamide is a carbonic anhydrase inhibitor, characterized by its unique sulfonamide group. It is a white, crystalline powder with a bitter taste and is soluble in water. Its ability to inhibit carbonic anhydrase makes it effective in reducing intraocular pressure and controlling seizures.
-
Mechanism of Action: Acetazolamide works by inhibiting the enzyme carbonic anhydrase, which plays a role in the regulation of fluid balance in the body. This inhibition reduces the production of aqueous humor in the eye, decreases seizure activity in the brain, and helps manage fluid retention.
-
Applications: It is primarily used to treat glaucoma by lowering intraocular pressure. Additionally, it is used as an adjunct in the treatment of epilepsy, to prevent and reduce the symptoms of altitude sickness, and to manage certain types of fluid retention (edema) associated with congestive heart failure or drug-induced edema.
-
Side Effects and Monitoring: Common side effects of Acetazolamide include fatigue, dizziness, and gastrointestinal disturbances. More serious side effects may include electrolyte imbalances and allergic reactions, requiring careful monitoring of patients, especially those on long-term therapy.
Key Market Drivers
Several factors are driving the demand for Acetazolamide in the global market. Understanding these drivers is crucial for stakeholders looking to capitalize on growth opportunities in this sector:
-
Increasing Prevalence of Glaucoma: The global rise in the incidence of glaucoma, particularly among the aging population, is a significant driver for Acetazolamide. Its effectiveness in reducing intraocular pressure makes it a preferred treatment option.
-
Rising Incidences of Altitude Sickness: As travel to high-altitude destinations becomes more popular, the demand for effective treatments for altitude sickness, such as Acetazolamide, continues to grow.
-
Expanding Applications in Epilepsy Management: Acetazolamide is used as an adjunctive therapy in the management of epilepsy, especially in cases resistant to conventional treatments. This application broadens its market scope.
-
Growth in Healthcare Infrastructure: Expansion of healthcare infrastructure and increased access to medical services, especially in emerging markets, contribute to the rising demand for Acetazolamide.
-
Regulatory Approvals and Market Expansion: Ongoing regulatory approvals for new indications and formulations of Acetazolamide, along with strategic market expansions by pharmaceutical companies, facilitate its availability and use in various regions.
Raw Materials Requirements
The production of Acetazolamide involves several key raw materials, each contributing to the final product’s quality and production cost:
-
Acetic Anhydride: This reagent is essential for the acylation step in the synthesis of Acetazolamide. The purity and availability of acetic anhydride directly impact the efficiency and cost of production.
-
Ethyl Cyanoacetate: Used in the initial condensation reaction, ethyl cyanoacetate is a crucial intermediate in the synthesis process. Ensuring a consistent supply of high-quality ethyl cyanoacetate is vital for maintaining production yields and product quality.
-
Aromatic Amines: These compounds form the core structure of Acetazolamide and are critical in the cyclization step of its synthesis. The quality and consistency of aromatic amines are essential for the stability and efficacy of the final product.
-
Solvents and Catalysts: Various organic solvents and chemical catalysts are required for the synthesis and purification of Acetazolamide. These include aprotic solvents and base catalysts that facilitate the chemical reactions involved in the production process.
-
Energy and Utilities: Significant energy inputs are needed for heating, cooling, and maintaining the reaction conditions during synthesis. Efficient energy management and sourcing strategies are essential for cost control and sustainability in production.
Costs and Key Process Information
Understanding the cost structure and key processes involved in Acetazolamide production is essential for evaluating the feasibility and profitability of manufacturing this medication. Here are the primary considerations:
-
Capital Expenditures (CapEx): Initial investments include setting up synthesis and purification facilities, purchasing advanced equipment, and establishing infrastructure for raw material handling and storage. The scale and technology of the production plant significantly influence the CapEx.
-
Operational Expenditures (OpEx): Ongoing costs encompass raw material procurement, energy consumption, labor, maintenance, and compliance with regulatory standards. Efficient production processes and economies of scale can help in reducing these costs.
-
Synthesis and Purification Process: The production process typically involves multi-step chemical synthesis, including condensation, cyclization, and purification stages. Each step requires precision and expertise to ensure the final product meets the required quality standards.
-
Product Quality and Yield: The quality of Acetazolamide is determined by its purity, potency, and consistency. Achieving high yield and maintaining product quality are crucial for meeting regulatory standards and market demands.
-
Regulatory Compliance: Adhering to Good Manufacturing Practices (GMP) and other pharmaceutical regulations is essential. Compliance ensures the production of safe and effective Acetazolamide, which is critical for gaining market approval and consumer trust.
Looking for an Exhaustive and Personalized Report?
Navigating the complex landscape of Acetazolamide production and its market applications requires access to comprehensive, accurate, and tailored insights. Whether you are a manufacturer, investor, or stakeholder in the pharmaceutical industry, a detailed report customized to your specific needs can significantly support your business decisions.
-
Customized Market Insights: Obtain detailed analysis of market trends, drivers, and forecasts aligned with your business objectives. Understand how shifts in the market could impact your operations and identify potential growth opportunities.
-
In-depth Cost Analysis: Gain a thorough understanding of the cost structure involved in Acetazolamide production, including CapEx and OpEx. This analysis aids in financial planning, investment strategies, and cost optimization.
-
Supply Chain Evaluation: Assess your raw material sourcing and supply chain strategies to ensure efficiency and cost-effectiveness. Identify and address potential supply chain bottlenecks and risks.
-
Regulatory and Compliance Insights: Stay compliant with evolving pharmaceutical regulations and implement sustainable practices. These insights help you manage compliance costs and enhance your market positioning.
-
Technological Advancements: Keep updated with the latest technological developments in Acetazolamide production that can improve your production efficiency and reduce costs.
Conclusion
Acetazolamide (Diamox) is a versatile medication used in the treatment of various medical conditions, including glaucoma, epilepsy, and altitude sickness. Understanding the production processes, market drivers, cost structures, and raw material requirements is vital for businesses looking to succeed in this competitive pharmaceutical sector.
For stakeholders in the Acetazolamide industry, investing in detailed market insights and cost analysis is essential for making informed, strategic decisions. Whether you are looking to expand your production capabilities, optimize your supply chain, or explore new market opportunities, a comprehensive and personalized report can significantly support your business strategy.
If you’re seeking exhaustive and tailored insights into the Acetazolamide market, our team of experts is here to provide the information you need. We offer customized assessments that help you navigate the complexities of Acetazolamide production and capitalize on the opportunities in this critical market.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Amanda Williams
Email: sales@procurementresource.com
Toll-Free Number: USA Canada – Phone no: +1 307 363 1045 | UK – Phone no: +44 7537 132103 | Asia-Pacific (APAC) – Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA














