Unlocking the Secrets of the Periodic Table of Elements
Share this Post to earn Money ( Upto ₹100 per 1000 Views )
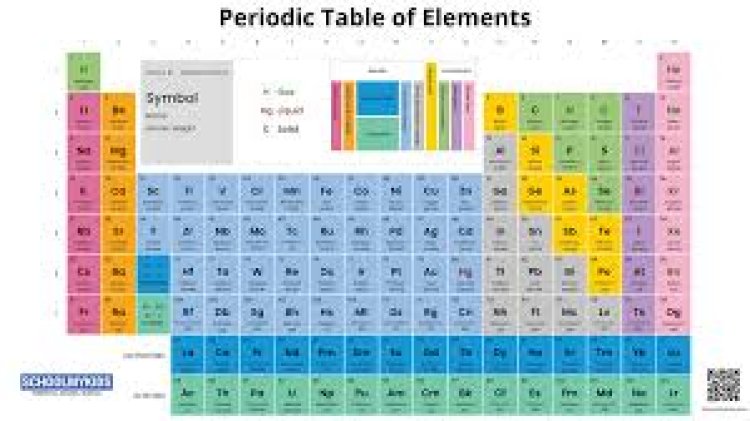
The periodic table of elements is one of the most important tools in the field of chemistry. It organizes all known chemical elements in a systematic way, providing essential information that helps scientists understand the properties and behaviors of these fundamental building blocks of matter.
What is the Periodic Table of Elements?
The periodic table of elements is a tabular arrangement of the chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties. Elements are listed in order of increasing atomic number, which corresponds to the number of protons in the nucleus of each atom. This structure not only helps identify each element but also indicates relationships between them.
Key Features of the Periodic Table
-
Groups and Periods: The table is divided into rows called periods and columns known as groups or families. Elements in the same group often exhibit similar chemical behaviors due to having the same number of valence electrons.
-
Metals, Nonmetals, and Metalloids: The periodic table categorizes elements into metals, nonmetals, and metalloids. Metals, located on the left side of the table, are typically shiny and good conductors of heat and electricity. Nonmetals, found on the right, have varied properties and are often poor conductors. Metalloids, located between metals and nonmetals, have characteristics of both.
-
Atomic Number and Mass: Each element in the periodic table is represented by its chemical symbol, atomic number, and atomic mass. The atomic number uniquely identifies the element, while the atomic mass reflects the average mass of its isotopes.
-
Lanthanides and Actinides: The two rows at the bottom of the periodic table are known as the lanthanide and actinide series. These elements are often placed separately to keep the table more compact and organized.
The Importance of the Periodic Table
The periodic table of elements serves as a vital reference for chemists, physicists, and students alike. Understanding how elements interact based on their positions in the table can help predict chemical reactions, discover new materials, and even advance technology.
-
Chemical Reactions: The arrangement of elements helps predict how they will react with one another. For instance, elements in the same group tend to react similarly because they have similar electron configurations.
-
Educational Tool: For students, the periodic table is an essential learning tool. It lays the foundation for understanding chemical principles and the nature of matter, making it easier to grasp more complex scientific concepts.
Resources for Learning About the Periodic Table
For those interested in diving deeper into the periodic table of elements, there are numerous resources available:
-
Online Platforms: Websites like Schoolmykids provide comprehensive information about the periodic table, including detailed descriptions of each element, their properties, and their applications.
-
Interactive Learning: Engaging with interactive periodic tables online can enhance understanding, allowing students to explore various aspects of elements through clickable interfaces.
-
Textbooks and Workshops: Consider enrolling in chemistry courses or workshops that focus on the periodic table, providing a more structured learning environment.
Conclusion
The periodic table of elements is not just a collection of symbols and numbers; it is a powerful tool that unlocks the secrets of chemistry and the nature of matter. By understanding the periodic table of elements, students and enthusiasts alike can gain insights into the world around them and appreciate the intricate relationships between different substances. Resources like SchoolMyKids offer valuable information to further explore this essential topic, making learning about chemistry both informative and enjoyable.















