Procedure for Rolling Out a Document Management System in Your Office
Share this Post to earn Money ( Upto ₹100 per 1000 Views )
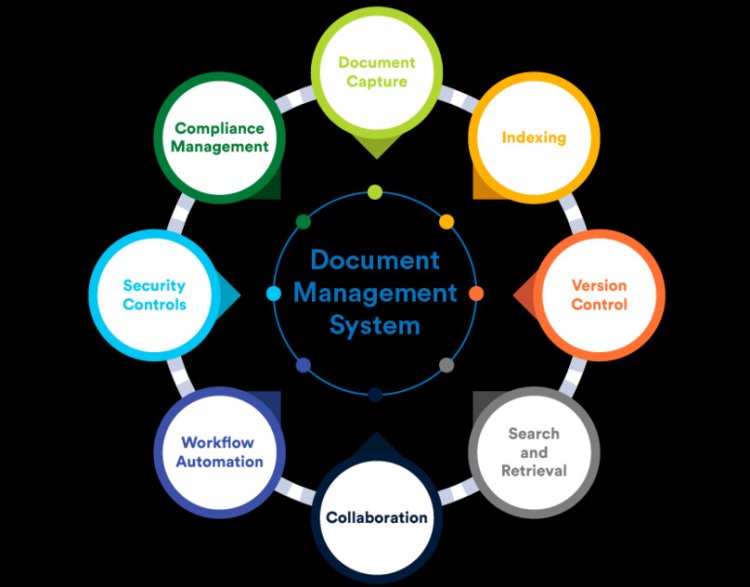
In today's fast-paced business environment, especially within the Life Sciences and Manufacturing sectors, effective document management is crucial. Implementing a document management system (DMS) can streamline operations, enhance compliance, and boost overall productivity.
Recognizing Why a Document Management System Matters
A document management system is more than just a digital storage solution; it is a strategic tool that helps organizations manage, store, and track electronic documents and images of paper-based information. For companies in highly regulated industries like Medical Devices, Pharma, Automotive, and Aerospace & Defense, a robust DMS ensures compliance with industry standards and regulations. By adopting a document management system, businesses can reduce errors, improve collaboration, and maintain a single source of truth for all critical documents.
The Role of EDMS in Modern Offices
Electronic Document Management Systems (EDMS) play a pivotal role in transforming how organizations handle their documents. An EDMS provides features such as version control, access permissions, and audit trails, which are essential for maintaining document integrity and security. For Quality Assurance Managers and CEOs, an EDMS ensures that all documents are up-to-date and accessible, facilitating better decision-making and regulatory compliance.
Choosing the Right Cloud-Based DMS for Your Business
Cloud-based DMS solutions offer unparalleled flexibility and scalability, making them ideal for businesses looking to modernize their document management processes. Unlike traditional on-premises systems, cloud-based DMS can be accessed from anywhere, promoting remote work and collaboration across different geographical locations. This is particularly beneficial for companies operating in multiple countries, such as the US, Canada, UK, Germany, and Australia, as it ensures seamless access to documents regardless of where employees are based.
Benefits of Cloud-Based DMS
Adopting a cloud-based DMS brings numerous advantages, including cost savings on infrastructure, automatic updates, and enhanced security features. Cloud-based DMS providers typically offer robust data encryption and backup solutions, ensuring that your documents are protected against data loss and cyber threats.
Implementing Document Control Software: A Step-by-Step Guide
Document control software helps organizations manage the lifecycle of their documents, from creation and approval to distribution and archiving. This ensures that all documents are accurate, up-to-date, and compliant with regulatory requirements.
Step 1: Scrutinize Your Present Document Management Strategies
Before implementing document control software, it is essential to evaluate your existing document management practices. Identify the pain points, such as inefficiencies in document retrieval, lack of version control, or compliance challenges. Understanding your current processes will help you determine the specific features and functionalities you need in a document control software solution.
Step 2: Define Your Document Management Requirements
Clearly outlining your document management requirements is crucial for selecting the right document control software. Consider factors such as the types of documents you manage, the level of security required, integration with existing systems, and user accessibility. For organizations in the Life Sciences and Manufacturing sectors, ensuring compliance with industry-specific regulations should be a top priority when defining your requirements.
Step 3: Select a Document Control Software Vendor
Selecting the appropriate supplier is essential for the effective deployment of your document management system. Seek out providers that deliver all-encompassing document control solutions, incorporating functionalities such as automated workflow processes, detailed audit logs, and powerful search features.
Integrating EDMS with Existing Quality Management Systems
Integrating an EDMS with your existing quality management systems (QMS) can significantly enhance your organization's ability to maintain high standards of quality and compliance. Seamless integration ensures that all quality-related documents are easily accessible and managed within a centralized system, reducing the risk of errors and improving overall efficiency.
Benefits of Integration
Integrating EDMS with QMS allows for better tracking of document revisions, automated approval processes, and real-time monitoring of compliance metrics. This integration helps Quality Assurance Managers ensure that all quality documents are current and compliant with regulatory standards, thereby minimizing the risk of non-compliance and associated penalties.
Training and Adoption: Attaining Optimal Results with Your Document Control Platform
Successful implementation of a document management system requires more than just installing the software; it involves ensuring that all users are adequately trained and that the system is adopted across the organization. Effective training programs and change management strategies are essential to drive user adoption and maximize the benefits of your document management system.
Developing a Training Plan
Create a comprehensive training plan that includes hands-on training sessions, user manuals, and ongoing support. Tailor the training to different user roles, ensuring that everyone understands how to use the system effectively and how it benefits their specific functions. Encourage feedback and continuously improve the training program based on user experiences and needs.
Promoting User Adoption
Promote user adoption by highlighting the efficiency gains and improved document accessibility that come with the new system. Recognize and address any concerns or resistance by involving key stakeholders in the implementation process and demonstrating the tangible benefits of the document management system. Providing continuous support and celebrating milestones can also help foster a positive attitude towards the new system.
Conclusion
In the dynamic and highly regulated landscapes of Life Sciences and Manufacturing, implementing a robust document management system is not just a strategic advantage but a necessity. ComplianceQuest stands out as an essential solution for businesses in 2024, offering advanced document management capabilities tailored to meet the stringent requirements of regulated industries. By leveraging ComplianceQuest, organizations can ensure seamless integration with their quality management systems, enhance compliance and security, and drive operational excellence.















