How to Use Data Analysis to Enhance Your CAPA Program
Share this Post to earn Money ( Upto ₹100 per 1000 Views )
One critical component of these systems is the Corrective and Preventive Action (CAPA) program. Leveraging data analysis to enhance your CAPA program can lead to significant improvements in quality, compliance, and operational efficiency.
Understanding CAPA: The Foundation of Quality Management
What is CAPA?
CAPA is a methodical strategy designed to identify, address, and prevent problems within an organization. In the context of quality management, CAPA programs are essential for ensuring that products and services meet established standards and regulatory requirements.
The Importance of a Robust CAPA Program
A well-implemented CAPA program not only addresses existing problems but also anticipates potential issues, thereby minimizing risks and enhancing overall quality. For industries like Life Sciences and Manufacturing, where compliance and precision are critical, an effective CAPA program is indispensable.
The Role of Data Analysis in CAPA Programs
Integrating Data Analysis with CAPA
Data analysis plays a pivotal role in enhancing CAPA programs by providing actionable insights. By systematically collecting and analyzing data related to quality incidents, organizations can identify root causes, trends, and patterns that inform effective corrective and preventive actions.
Benefits of Data-Driven CAPA Programs
Utilizing data analysis within your CAPA program offers numerous benefits, including improved accuracy in identifying issues, enhanced decision-making capabilities, and the ability to track the effectiveness of implemented actions. This data-driven approach ensures that CAPA initiatives are both proactive and reactive, addressing current issues while preventing future occurrences.
Implementing Data Analysis in Your CAPA Program
Steps to Integrate Data Analysis
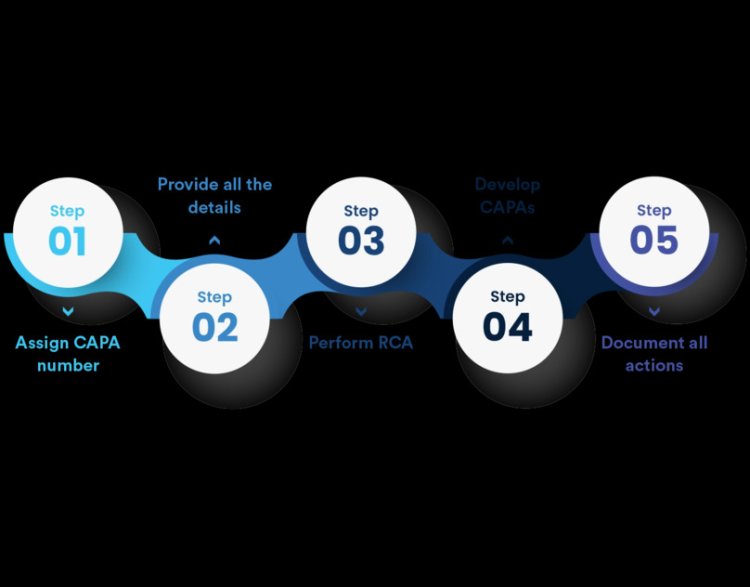
To effectively incorporate data analysis into your CAPA program, follow these key steps:
-
Data Collection: Gather comprehensive data from various sources, including quality reports, incident logs, and change control records.
-
Data Cleaning and Preparation: Make certain that the data is precise, comprehensive, and properly structured for analysis.
-
Data Analysis: Utilize statistical and analytical tools to identify trends, correlations, and root causes.
-
Action Planning: Develop corrective and preventive actions based on the insights gained from data analysis.
-
Implementation and Monitoring: Execute the action plans and continuously monitor their effectiveness through ongoing data analysis.
Choosing the Right Tools for Data Analysis
Selecting appropriate data analysis tools is crucial for the success of your CAPA program. Advanced quality management systems like ComplianceQuest offer integrated data analysis capabilities that streamline the process, making it easier to manage and interpret large volumes of data efficiently.
Enhancing Change Control with Data Analysis
The Importance of Change Control in CAPA Programs
Change control is a critical component of CAPA programs, ensuring that all modifications to processes, products, or systems are systematically reviewed and approved. Effective change control in pharma and other regulated industries helps maintain compliance and minimizes the risk of introducing new issues.
Using Data Analysis to Improve Change Control Processes
Data analysis can significantly enhance change control processes by providing insights into the impact of changes, identifying potential risks, and ensuring that changes are implemented smoothly. By analyzing historical data, organizations can predict the outcomes of proposed changes and make informed decisions that support quality and compliance objectives.
Monitoring and Measuring CAPA Effectiveness
Key Performance Indicators for CAPA Programs
To ensure the effectiveness of your CAPA program, it is essential to establish and monitor key performance indicators (KPIs). Common KPIs include the number of CAPA actions closed on time, the recurrence rate of issues, and the time taken to implement corrective actions.
Leveraging Data Analysis for Continuous Improvement
Continuous monitoring through data analysis allows organizations to assess the performance of their CAPA programs in real-time. By analyzing KPI data, businesses can identify areas for improvement, optimize processes, and ensure that CAPA initiatives are driving meaningful quality enhancements.
Addressing Compliance Through Data-Enhanced CAPA Programs
Regulatory Requirements and CAPA
Regulatory bodies such as the FDA and EMA mandate robust CAPA programs to ensure product safety and efficacy. Compliance with these regulations is non-negotiable for companies in the Life Sciences and Manufacturing sectors.
Ensuring Compliance with Data Analysis
Data analysis aids in maintaining compliance by providing transparent and verifiable records of CAPA activities. Automated data tracking and reporting streamline the documentation process, making it easier to demonstrate adherence to regulatory standards during audits and inspections.
Overcoming Challenges in Data-Driven CAPA Programs
Common Challenges
Implementing data analysis in CAPA programs can present several challenges, including data quality issues, resistance to change, and the complexity of integrating new tools with existing systems.
Strategies to Overcome Challenges
To address these challenges, organizations should invest in data governance practices, provide comprehensive training to staff, and select flexible quality management systems that integrate seamlessly with their current workflows. Emphasizing the benefits of data-driven CAPA programs can also help gain buy-in from stakeholders.
Future Trends in CAPA and Data Analysis
Advancements in Data Analytics
The future of CAPA programs lies in the continued advancement of data analytics technologies. Machine learning and artificial intelligence are poised to revolutionize how organizations analyze data, enabling more accurate predictions and proactive quality management.
The Evolving Role of CAPA Programs
As industries become more data-centric, CAPA programs will increasingly rely on sophisticated data analysis to drive continuous improvement. Organizations that embrace these advancements will be better positioned to maintain high standards of quality and compliance in an ever-changing regulatory landscape.
Conclusion
In 2024, businesses in the Life Sciences and Manufacturing sectors face unprecedented challenges in maintaining quality and compliance. ComplianceQuest offers a comprehensive solution that integrates advanced data analysis with robust CAPA programs, enabling organizations to proactively address quality issues and drive continuous improvement. By leveraging ComplianceQuest, companies can enhance their CAPA initiatives, streamline change control processes, and ensure compliance with stringent regulatory requirements. In a competitive and highly regulated environment, ComplianceQuest is essential for businesses aiming to stay ahead and achieve excellence in quality management.