How to Foster Collaboration in Corrective and Preventive Action (CAPA) Processes
Share this Post to earn Money ( Upto ₹100 per 1000 Views )
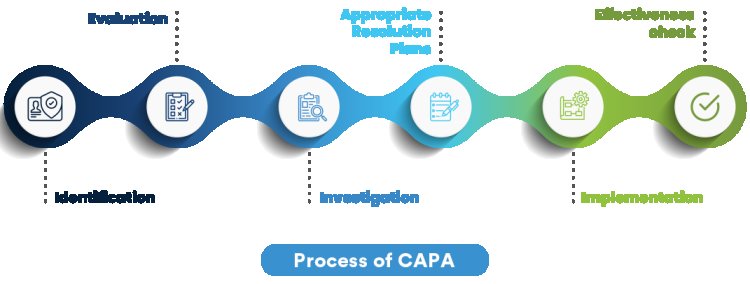
In the highly regulated industries of Life Sciences and Manufacturing, the Corrective and Preventive Action (CAPA) process plays a pivotal role in ensuring product quality, safety, and regulatory compliance. However, to maximize the effectiveness of CAPA, it is crucial to foster collaboration across various teams and departments. This collaborative approach not only improves the CAPA process but also strengthens the overall quality management framework within an organization.
1. Understanding the Importance of Collaboration in CAPA
Collaboration is a cornerstone of an effective CAPA program. In the complex environments of Life Sciences and Manufacturing, issues rarely arise in isolation. Therefore, addressing these issues requires the input and expertise of multiple departments, including quality assurance, production, engineering, and regulatory affairs. Collaboration ensures that all relevant perspectives are considered, leading to more comprehensive and effective corrective and preventive actions.
The Role of Cross-Functional Teams in CAPA
Cross-functional teams bring together diverse skills and knowledge, enabling a more thorough analysis of the root causes of non-conformances. By fostering collaboration among these teams, organizations can ensure that their CAPA processes are not only reactive but also proactive in preventing future issues.
2. Building a Collaborative CAPA Program
A well-structured CAPA program should be designed to promote collaboration at every stage. This includes the identification of potential issues, the investigation of root causes, the implementation of corrective actions, and the monitoring of their effectiveness.
Establishing Clear Communication Channels
Effective communication is the backbone of a collaborative CAPA program. Organizations should establish clear communication channels that allow for the seamless exchange of information between departments. This can be facilitated through regular meetings, shared digital platforms, and the use of change management software to track and manage CAPA activities.
3. Leveraging Change Management to Enhance CAPA Collaboration
Change Management is integral to the success of CAPA processes, particularly when corrective actions involve changes to existing processes, procedures, or systems. By integrating change management principles into the CAPA program, organizations can ensure that changes are implemented smoothly and that all stakeholders are on the same page.
The Role of Change Management Software in CAPA
Change management software provides a centralized platform for managing changes related to CAPA. It enables organizations to track the progress of changes, document approvals, and ensure that all relevant parties are informed and involved. This level of transparency and coordination is essential for fostering collaboration in CAPA processes.
4. Encouraging a Culture of Collaboration in CAPA Processes
Beyond the structural and technological aspects, fostering collaboration in CAPA processes also requires a cultural shift within the organization. Leaders must encourage a culture where collaboration is valued and where team members feel empowered to contribute to the CAPA process.
Leadership's Role in Promoting Collaboration
By actively promoting a collaborative culture and providing the necessary resources, leaders can ensure that their teams are fully engaged in the CAPA process. This, in turn, leads to more effective problem-solving and continuous improvement.
5. Utilizing Technology to Support Collaborative CAPA Efforts
Technology, particularly Change Management Software, can greatly enhance collaboration in CAPA processes. These tools provide a framework for managing CAPA activities, ensuring that all team members have access to the information they need and can contribute effectively.
Integrating CAPA and Change Management Tools
The integration of CAPA and change management tools allows for a more streamlined approach to managing corrective actions. By leveraging technology, organizations can ensure that their CAPA processes are both efficient and collaborative, ultimately leading to better outcomes.
6. Overcoming Barriers to Collaboration in CAPA
While collaboration is essential, it is not always easy to achieve. Organizations may face barriers such as departmental silos, lack of communication, and resistance to change. Addressing these barriers is critical to fostering a collaborative CAPA environment.
Strategies for Breaking Down Silos
Breaking down departmental silos requires a concerted effort to promote cross-functional collaboration. This can be achieved through team-building activities, cross-training, and the use of collaborative technologies that facilitate communication and information sharing across departments.
7. Monitoring and Improving Collaborative CAPA Processes
Once a collaborative CAPA process is established, it is important to continuously monitor and improve it. Regular reviews, feedback sessions, and audits can help identify areas for improvement and ensure that collaboration remains strong.
The Role of Continuous Improvement in CAPA
Continuous improvement is at the heart of a successful CAPA program. By fostering a culture of continuous learning and improvement, organizations can ensure that their CAPA processes remain effective and that collaboration continues to thrive.
8. Case Studies: Successful Collaboration in CAPA
To illustrate the importance of collaboration in CAPA, consider the following case studies from the Life Sciences and Manufacturing sectors. These examples highlight how organizations have successfully fostered collaboration in their CAPA programs, leading to improved quality outcomes and regulatory compliance.
Lessons Learned from Collaborative CAPA Efforts
These case studies provide valuable insights into the best practices for fostering collaboration in CAPA processes. They demonstrate the importance of clear communication, the use of change management software, and the role of leadership in promoting a collaborative culture.
Conclusion
In 2024, the need for robust, collaborative CAPA processes is more critical than ever. As organizations in the Life Sciences and Manufacturing sectors face increasing regulatory scrutiny and market pressures, the ability to effectively manage corrective and preventive actions will be a key differentiator. ComplianceQuest Management Software provides the tools and framework necessary to foster collaboration, integrate change management, and drive continuous improvement in CAPA processes. By investing in ComplianceQuest, organizations can ensure that they are not only meeting regulatory requirements but also achieving operational excellence.















